Immunotherapy is one call away Call Now

Advanced Targeted Immunotherapy Options Enhance Precision Cancer Treatment
Your immune system is the first and last defense against cancer. Immunotherapy strengthens these defenses by bolstering your immune system to give you a greater chance at achieving treatment success.
Envita Medical Centers, a Center of Excellence for Precision Oncology, has been focusing on harnessing the power of the immune system to heal our patients holistically. We understand that when people have cancer, their immune system gets compromised. Over time, the immune cells, such as the NK and DC cells, become less responsive so the cancer cells find ways to evade the immune system, causing an uncontrolled growth and spread of the disease. To overcome this challenge, Envita goes far beyond typical immunotherapy drugs, such as PD-1 inhibitors, building next level immuno reactivation treatment plans for our patients.

When patients become resistant to treatment, immunotherapy can bring about immuno-reactivation, which helps them respond to care.
It is critical to be cautious about the term immunotherapy, as it only defines a category of treatment, without going into its varied levels and types which are essential to gauge its depth. Most patients have little to no access to immunotherapy for their cancer treatment, let alone the customization provided by Envita. We deliver next-level custom targeting, offering the benefits of the highest level and multiple types of immunotherapies to our patients.
With our 25 years of experience treating complex and late-stage cancer cases, we have developed sophisticated algorithms to integrate genetically targeted immunotherapy options into our personalized and comprehensive cancer treatment protocols. Strengthening the immune system is a crucial part of cancer treatment that is often overlooked in standard oncology or limited at best. Not having immunotherapy as a part of your cancer treatment protocol could be the missing piece limiting your treatment success.

Immunotherapy is the forerunner of changing the way we treat cancer.
Current standard oncology relies heavily on chemotherapy, radiation, and surgery, which may not be enough to shut down the cancer and can lead to further immune system suppression if not properly targeted. At Envita Medical Centers, we combine next-level immunotherapy options, leading-edge conventional treatments, and adjuvant care with the goal to improve patients’ quality of life, increase longevity, and reduce toxicity.
Several recent studies indicate how immunotherapy may aid in tackling two of the worst fears of every cancer patient, recurrence and cancer spread also known as metastasis [1].

Almost 90 percent of cancer deaths happen due to metastasis and resistance to chemotherapy, which is the result of cancer spreading, unchecked by the immune system, to different parts of the body making for an increasingly more challenging form of disease [2].

We wouldn’t have cancer if our immune system could seek, find, and kill our cancerous cells.
A healthy immune system kills 10,000 cancerous and precancerous cells every day, but when it does not function properly the cancer gets a chance to grow and spread. Standard oncology treatments, when not properly targeted for the patient’s genomic needs, may further suppress the immune system leading to cancer relapse, which is why including immunotherapy can be a gamechanger in cancer treatment. Reactivating the immune system is especially critical when patients exhibit high mutational loads and chemotherapy is no longer working.

Immunotherapy is the future of cancer treatment, but at Envita, the future is now! We have been doing this for the last 25 years and have been constantly innovating and evolving immunotherapy options for our patients.
Why Cancer Patients Need Immunotherapy Now
Immuno Targeting Invokes a Multi-Dimensional Attack on Cancer
Recent studies on genes and genetic mutations within cancer cells have revealed a fact that Envita has known to be true for nearly two decades; all cancer patients’ genetic markers and mutations are unique to the individual. The differing genetic markers of the cancer explains why patients with the same type and stage of cancer do not respond similarly to receiving the same treatment. Because cancer is a disease of genetic mutations and each patient’s genetics are different, their molecular profiles and biomarkers, which reveal treatment targets, will vary.
Biomarkers are attributes of cancer cells containing specific chemotherapy and immunotherapy targets. By harvesting the latest biomarker information through Envita’s Ultra Analytes Liquid Biopsy, our expert team of physicians can identify actionable targets for chemotherapeutic agents, adjuvant therapies, and immunotherapies your body is most likely to respond to. Immuno targeting with Envita’s Ultra Analytes Liquid Biopsy helps to direct a multi-dimensional attack on cancer cells to help you gain an edge over your disease.
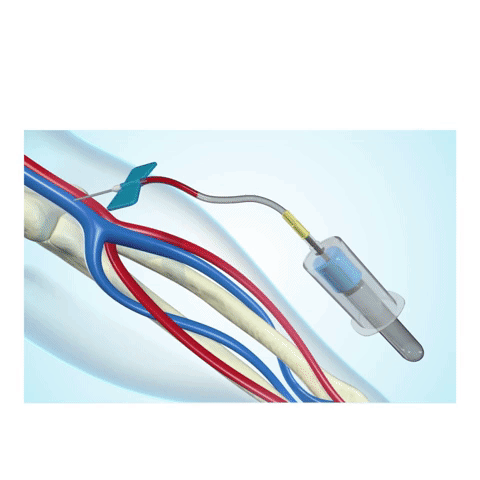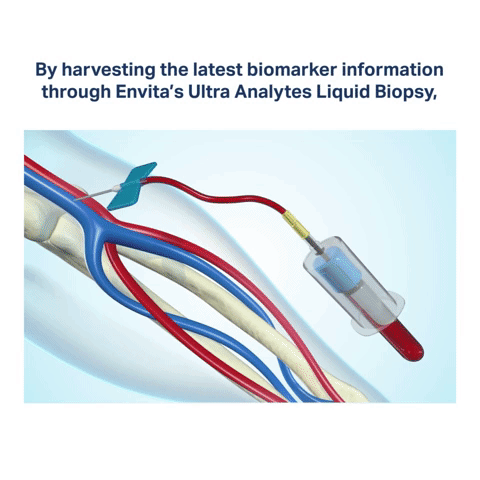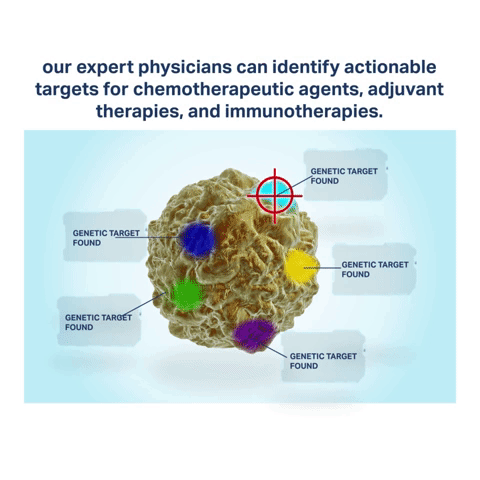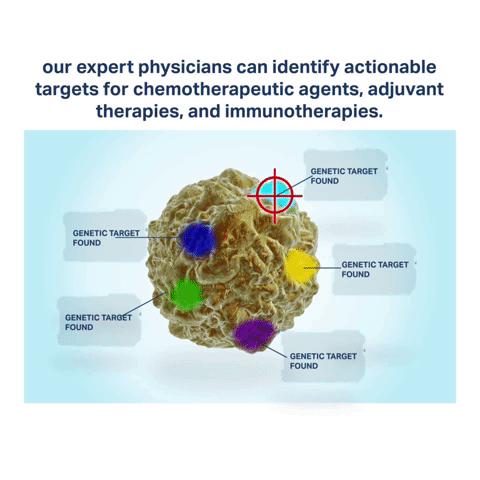
Biomarkers are attributes of cancer cells containing specific chemotherapy and immunotherapy targets. By harvesting the latest biomarker information through Envita’s Ultra Analytes Liquid Biopsy, our expert team of physicians can identify actionable targets for chemotherapeutic agents, adjuvant therapies, and immunotherapies your body is most likely to respond to. Immuno targeting with Envita’s Ultra Analytes Liquid Biopsy helps to direct a multi-dimensional attack on cancer cells to help you gain an edge over your disease.
Addressing the Challenges of Micro-Disease and Metastasis
Envita’s Ultra Analytes Liquid Biopsy allows us to examine the most recent mutations in the cancer cells by analyzing the CTCs (Circulating Tumor Cells) collected from a patient’s blood sample, as opposed to analyzing old tissue biopsy. A tissue biopsy is typically performed when a cancer is first diagnosed. When the patients stop responding to treatment, many standard oncology facilities will perform genomic analysis on this biopsied tissue to determine precision treatment options.
Often, these biopsies are harvested weeks or months before the patient failed treatment. Because cancer is a disease of mutations that is constantly changing, these original tissue biopsies may contain outdated information which may not lead to a proactive treatment. Our liquid biopsy attempts to overcome this challenge by identifying traces of micro disease and dormant metastasis via the fresh mutations in the CTCs.

Often, these biopsies are harvested weeks or months before the patient failed treatment. Because cancer is a disease of mutations that is constantly changing, these original tissue biopsies may contain outdated information which may not lead to a proactive treatment. Our liquid biopsy attempts to overcome this challenge by identifying traces of micro disease and dormant metastasis via the fresh mutations in the CTCs.
We can see where the cancer is spreading even when it is not visible in imaging. Armed with this information we are better equipped to establish targeted immunotherapies which can treat the disease even before it manifests itself as metastatic spread.

Immunotherapy is the most underutilized sphere of cancer treatment, but at Envita we have been using it for over two decades to enhance treatment efficacy. We don’t follow a one-size-fits-all approach, rather we personalize our immunotherapies to optimize benefits for each individual patient.
Overcoming Unresponsive Chemotherapy Regimens
Chemotherapy alone is not enough for many cancer patients, especially when the chemo was not properly targeted to your genomic mutations. The NCCN (National Comprehensive Cancer Network) guidelines, followed in standard oncology, recommends chemotherapy based on tumor type and stage, not on individual genomic mutations. As a result, many cancer patients may be unresponsive to their chemotherapy.
A 2004 study by researchers on the impact of chemotherapy on 5-year survival in American adults show poor results. While in some types of cancer like non-Hodgkin’s lymphoma, 40.3 percent of patients lived longer than 5 years with only chemotherapy as their treatment, in other types of cancers like stomach and colon, survival rates were as poor as 0.7 and 1.0 percent respectively. Considering the 5-year survival rates of patients with different types of cancers, on an average only 2.1 percent lived longer than the study period [3].
Though this study represents 2004 data, today with the advent of smart drugs patients are living a bit longer but are still receiving poor prognoses for late stage and complex cancers.
In 2016, NIH invested about $250,000,000 dollars to accelerate precision oncology, but it has been slow to fruition. Envita changes all that with next-level precision treatment and planning, opening a world of options for our patients.
To overcome the challenge of poor patient outcomes, at Envita we not only combine immunotherapy along with chemotherapy, but our chemotherapies are precision-targeted to suit your individualized form of cancer. The chemotherapy administered at Envita is not only genetically typed to your disease but is typically 10 to 20 percent of the dosage administered in standard chemotherapy regimens. The lower and metronomic dosage allows us to use multiple chemotherapies at the same time, while reducing potential side-effects.
With the chemotherapy toxicity being lower (due to low dosage), you are less likely to experience the side-effects related to a weakened immune system, as can be seen in untargeted chemotherapy. A strong immune system is better equipped to check further growth and spread of cancer, aiding in potentially reaching long-term remission.

Immunotherapy promotes a different method to kill cancer, completely independent of chemotherapy, yet it can also make chemotherapy more effective.
Independent Ways to Slow Down Cancer Cells
Cancer treatment involves not just killing the cancer cells or the tumor, but also curbing the mutations at a cellular level. At Envita, we use different immunotherapies to slow down the mutations of the cancer cells, which in turn helps the chemo medicines to better hit their targets, optimizing tumor kill.
Our GTFC™ (Genetically Targeted Fractionated Chemotherapy) is not only highly targeted and low dosed, but it also uses patient-specific chemo and immuno agents, which are administered intravenously, to enhance cancer kill. This personalized precision oncology treatment option utilizes 7 to 10 different mechanisms of cancer kill, which is more than double of untargeted chemotherapy.
At Envita, we understand that every patient’s cancer is unique because their genetics are different, so our expert team including oncologists, interventional radiologists, researchers, and pharmacists collaborate to design a medical blueprint, unique to each patient’s state of disease.
Envita Medical Centers Key Metrics
- Campus buildings in Scottsdale, Arizona
- 5
- Sq. ft. of medical space
- 70K
- Custom infusions performed per year
- 60K
- Highly trained employees
- 200+
- Annual inquiries
- 13K
Levels of Immunotherapy
In our clinical experience, including immunotherapy in our personalized precision oncology treatment plans have helped cancer patients of all types and stages. Depending on the stage of their cancer, they may need a different level of immunotherapy.
The Different Levels of Immunotherapy
You can always tell how advanced a cancer center or integrative cancer center is by recognizing the level of immunotherapy.
Intro-level: Diet and nutritional supplements to target and fortify the immune system. Also includes herbs and homeopathic.
Mid-level: Intravenous Vitamin C and other agents deliver nutrients into the blood stream for activating the immune system.
High-level: Modulate levels of various cytokines and messengers,enabling patients’ immune systems to fight against cancer.
Highest-level: Expand and grow the number of cancer-fightingcells to enhance tumor kill and reestablish the immune system.
Types of Immunotherapies
While the intro-level and mid-level of immunotherapies are available in many integrative cancer care centers, the high and highest levels are available in limited cancer hospitals offering specialized care. However, at Envita, we take immunotherapy to the next level by personalizing each of the therapies to better target every patient’s specific cancer cell biomarkers. In our clinical experience, precision targeting the immunotherapies enhances treatment efficacy, putting our patients on the path towards healing.
Types of High-level Immunotherapy:
Checkpoint Inhibitors Therapy
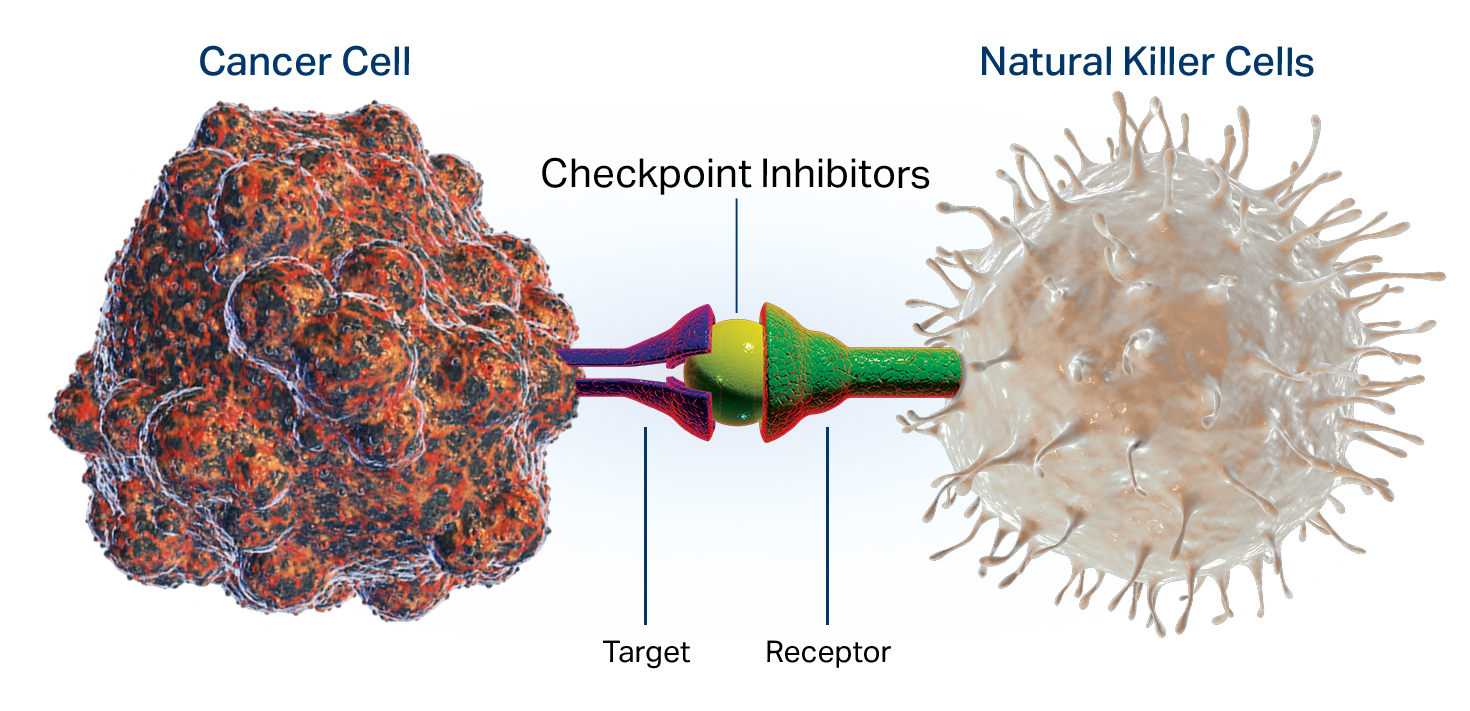
The immune system includes certain “brakes” called checkpoints, to prevent attack on our own healthy cells, while tackling other pathogens in the body. Cancer cells often manipulate these checkpoints to evade the immune system so they can grow and spread further. The checkpoint inhibitors, a type of smart drugs, are monoclonal antibodies made by researchers in the labs to release the “brakes” on your immune system.
These drugs block the proteins PD-1, PD-L1, and CTLA-4 on the surface of immune cells, allowing them to go after the cancerous cells. However, these drugs may not always work for everyone and in some cases, patients experience a rebound effect, as the cancer comes back within weeks, months, or years after they stop taking the drugs.
The Envita Difference: At Envita, we understand that rebounds happen because of the low cytotoxicity, toxicity caused due to the action of chemotherapeutic agents on living cells, of the natural killer (NK) cells in your immune system.
We know how to build and strengthen the NK cells, to make the checkpoint inhibitors work effectively.
Our sophisticated testing and algorithms allow us to go to the root of your individual cancer so we can utilize a combination of different smart drugs and other immunotherapies needed to optimize the efficacy of checkpoint inhibitors.
Immune System Modulators
Immune system modulators include therapies to influence the signaling and messaging used for instructing the immune system to go after the cancer. There are many components to the immune system which represent the body's army against disease. The immune systems functionality relies on signaling molecules, or cytokine proteins, which act as the medium through which cells communicate with one another.
Cytokines, such as, interleukins, interferons, and immunomodulators are responsible for boosting your immune system’s power in overcoming cancer. Generally, immune system modulator drugs are used to influence the immune system messaging in treating certain types of leukemia, sarcoma, lymphoma, melanoma, and advanced kidney cancer, but at Envita we custom-build a variety of these drugs to extend the benefits of immune system modulators to many other cancer patients.
The Envita Difference: Some integrative cancer centers administer vitamin C infusions and herbs to influence these cytokines but our specialized testing allows better understanding of the irregularities in this messaging.
With these insights we can unravel the messaging issues feeding the cancer growth, so we customize immunotherapies addressing your specific issues.

Envita creates Custom Immunotherapies
to treat your
specific cancer
Types of Highest-level Immunotherapy:
Cancer Vaccines
Cancer treatment vaccines are a type of immunotherapy, which trains the immune system to watch out for cancer cells so it can identify and kill them at the earliest opportunity. Certain genetically modified viruses or antigens, which are proteins expressed by the cancer cells, may be used to make these vaccines. The vaccines are injected into the patient to kill certain types of cancer cells and deactivate the tumor, but they may not have the desired effect on a ravaged immune system. However, with Envita’s personalized neoantigen vaccines and supportive adjunctive care, we can extend the benefits of cancer vaccines to reach a greater number of patients.
The Envita Difference: Neo-antigens are new antigens which are expressed exclusively by tumor cells and not by any of the patient’s healthy cells. With the right kind of intratumoral treatments, we can shut down the tumors and derive the neoantigens. These personalized neoantigen vaccines can overcome the challenges of standard vaccines which may sometime misdirect the immune system to attack its own healthy cells.
At Envita, our expert team has been working on personalized neo antigen vaccines since the early 2000s.
In our clinical experience, these vaccines have worked wonders for many of our patients with complex and late-stage cancers.
Adoptive Cell Therapies
In adoptive cell immunotherapy, some of your own immune cells are removed to either boost their numbers or genetically modify them in a lab so they can identify and kill cancer cells. Some such therapies are still in the clinical trial phase, while the CAR T-cell therapy is FDA-approved for treating people with some types of leukemia or lymphoma, in certain age groups. As part of the CAR T-cell therapy, special receptors are added to the surface of the patient’s T cells, a type of white blood cells which are a part of the immune system, so they can find and kill your specific type of cancer cells.
The limitation of this therapy lies in the fact that at the moment, only a few types of cancer patients in specific age groups are eligible for it because extending it beyond this group may trigger adverse consequences, such as autoimmune diseases. A 2016 study, published in the Journal of Cellular Immunotherapy, suggests that modifications of the immune system to safely treat cancer may include more than one type of T cell modification to balance potential adverse events from CAR T cell therapy [4].
The Envita Difference: At Envita, we try to overcome this challenge by using a combination of immunotherapies and personalizing them as needed to especially target each patient’s individual cancer cell mutations.
Envita’s IMX (Immune Modulation Treatment) is an autologous adoptive immunotherapy, which includes a combination of four different types of personalized immunotherapies designed to complement each other, mitigating potential treatment side effects, so patients may aim towards a holistic recovery.
IMX is not FDA-approved for cancer treatment in the United States, but we can do this procedure for patients who need it, in our international center located in Hermosillo, Mexico.
The four steps of IMX include, gene therapy, dendritic genetic peptide treatment, antigen activated NK expansion, and checkpoint inhibitors. As part of gene therapy, we use oncolytic viruses, which are nonpathogenic viruses, to kill the tumor. To prevent further growth and spread of the cancer, the antigens released by the tumor harness other parts of the immune system to identify and kill cancer cells, but in certain late-stage cancers the immune system is so weak that it is unable to check further cancer proliferation.
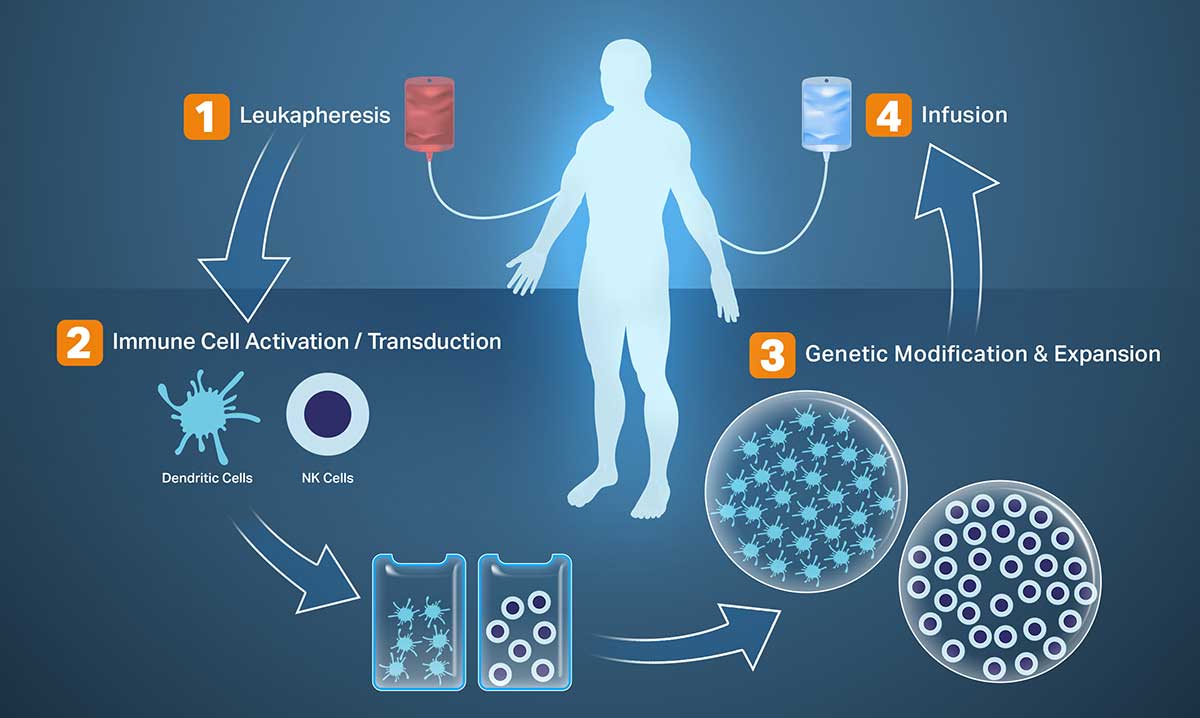
In such cases, we may need the dendritic genetic peptide treatment, the antigen activated NK expansion, or both to teach your immune system to effectively go after your cancer. We take the dendritic, NK, or both types of cells outside your body, genetically type them and grow them in our labs so they are powerful enough to identify and attack your specific cancer cells. A reactivated and strengthened immune system will still need checkpoint inhibitors to ensure the cancer cells don’t block the immune cells from going after the cancer and killing it.
Not all patients need to go through each of the four steps of IMX but depending on the complexity of the cancer and the status of the immune system, some patients may need the dendritic genetic peptide treatment and antigen activated NK expansion to tie together the benefits of gene therapy and checkpoint inhibitors. Not all patients are candidates for IMX.
Disclaimer: Some of the treatments mentioned (like IMX) in the above article are not all available in the US and are not FDA approved for the treatment or diagnosis of cancer. Patients should consult with their doctors before undergoing treatment or altering medical care. Envita Mexico is under Mexican law and jurisdiction and in no way does Envita make guarantees or claims of any outcomes for this or any treatment. In cancer treatment, individual results will vary, and outcomes should not be expected. The information above is for education purposes only. Not all patients are candidates for IMX or immunotherapies and should consult their doctors.
Is Everyone Eligible for Immunotherapy?
Everyone is eligible for immunotherapy of some level for most cancers, because in our clinical experience, it optimizes the efficacy of your treatment. Depending on the complexity of your cancer, our expert team decides which level or type of immunotherapy will be suitable for your unique cancer.
Some types of immunotherapies may trigger certain side-effects, so personalizing the immunotherapy is crucial to maximize its benefits, which is why at Envita we don’t follow the one-size-fits-all approach in immunotherapy. Our personalized precision oncology focuses on customizing advanced immunotherapies to reduce chances of side-effects and help patients progress towards remission.
To learn more about immunotherapy or to get started with treatment at Envita Medical Centers, please contact our team of patient care experts at 866-830-4576. These experts are standing by to answer any questions you may have and may God bless you on your journey to healing.
References
[1] Cha JH, Chan LC, Song MS, Hung MC. New Approaches on Cancer Immunotherapy. Cold Spring Harb Perspect Med. 2020;10(8):a036863. Published 2020 Aug 3. doi:10.1101/cshperspect.a036863
[2] Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18(1-2):43-73. doi:10.1615/critrevoncog.v18.i1-2.40
[3] Morgan, G., Ward, R., & Barton, M. (2004). The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clinical oncology (Royal College of Radiologists (Great Britain)), 16(8), 549–560. https://doi.org/10.1016/j.clon.2004.06.007
[4] Aaron J. Smith, John Oertle, Dan Warren, Dino Prato, Chimeric antigen receptor (CAR) T cell therapy for malignant cancers: Summary and perspective, Journal of Cellular Immunotherapy, Volume 2, Issue 2, 2016, Pages 59-68, ISSN 2352-1775, https://doi.org/10.1016/j.jocit.2016.08.001.
Choose Envita Medical Centers
Our team is ready to help you get your life back! Please enter your contact information and a Patient Care Expert will contact you shortly. If you would rather speak now, please give us a call at 1-866-830-4576 .

